生物技术和制药行业的过程分析技术
生物制药生产的特点在于使用现代技术、利用科学进步和高度复杂的研发工作
数十年来,Knick 一直在过程分析技术领域为全球企业提供咨询和支持。公司的核心技能包括可靠测量 pH 值、氧化还原、电导率和溶解氧。除了传感器之外,Knick 也提供创新的工业变送器、高质量的过程适配器和连接件以及独特的清洁和校准系统。

由高度复杂的研发驱动的先进技术
开发一种新型药物有效成分通常需要大量时间和资金,才能将科学发现转化为新药,并运用相应的生产资料建造专业生产设备。首先要生产第一批临床研究用的试验药物,获得 FDA 批准之后才能开始全面生产。
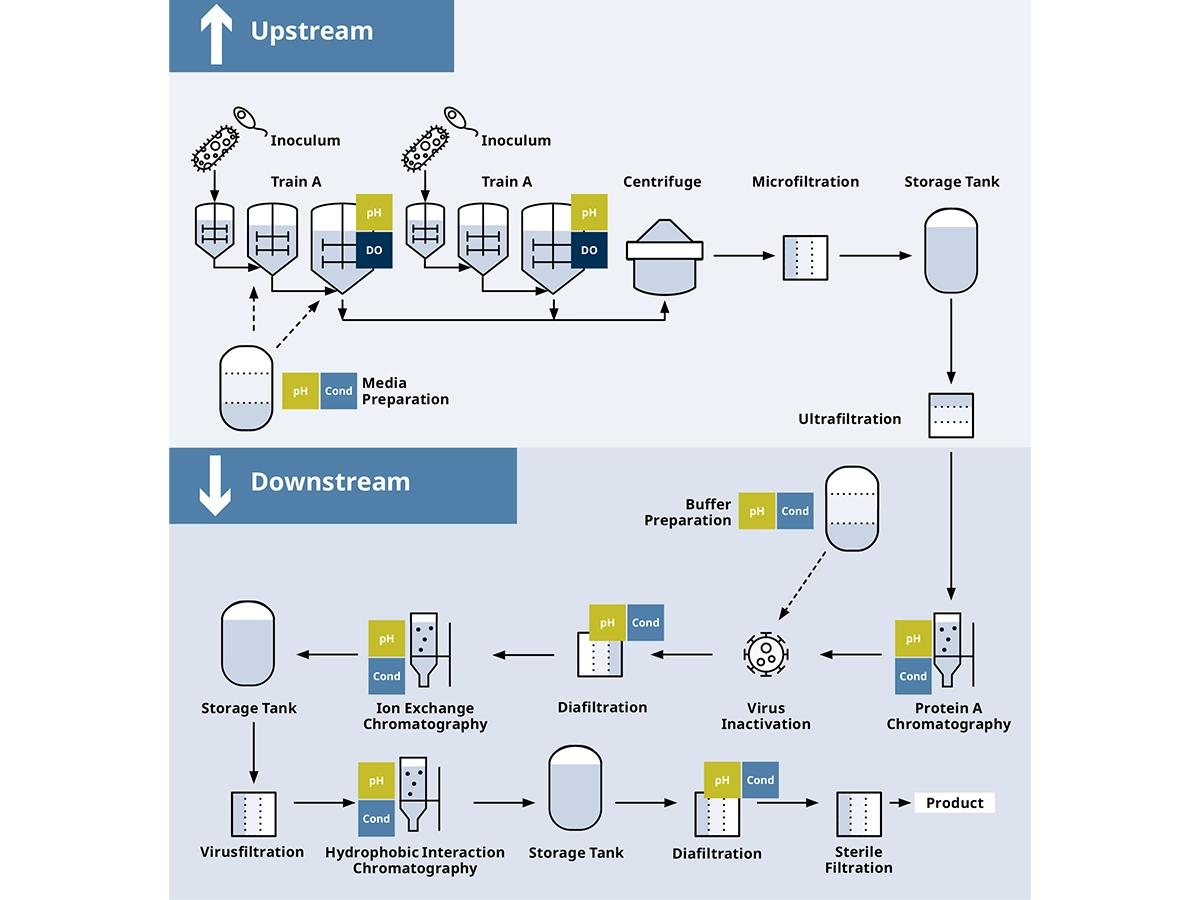
现在,如果没有生物制药行业开发的疗法,几乎不可能治疗罕见或复杂的疾病。可对动物和昆虫细胞以及细菌和酵母进行基因改造,以生产重组蛋白之类的靶分子、疫苗等。由于生物体的异质性,生产是一个复杂的过程。上游过程 (USP) 中的异质性也会转移到下游过程 (DSP) 中的产品净化步骤。
所有上游、下游和辅助过程的自动化有助于降低人为错误的风险,提高运行效率,从而提高最终产品的安全性和质量。
为何选择 Knick?
作为测量和控制技术专家,Knick 在复杂应用中的液体分析领域拥有高水平的能力和应用知识。自身的垂直一体化能确保产品质量和灵活性,以满足客户的具体要求。
公司贴近客户、诚信可靠,时至今日,我们的创新产品和技术备受全球化工和制药行业知名制造商的青睐。
生物制药过程:为每个过程步骤打造的解决方案
在生物制药行业,每个过程步骤都很重要,需要专门的测量技术来控制关键参数,以实现最大收率并尽可能减小偏差。
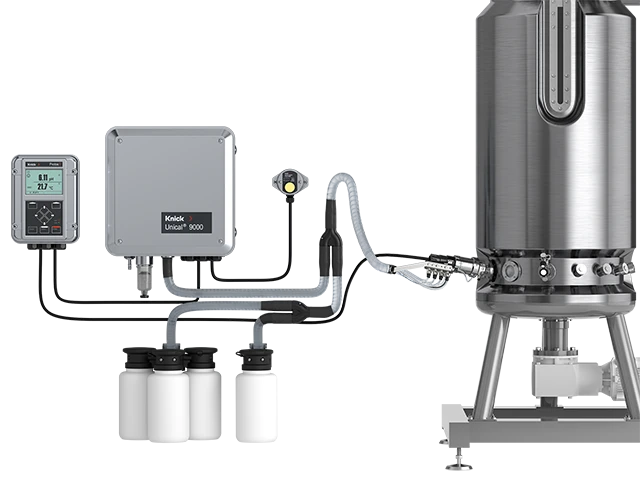
从传感器到全自动传感器维护系统,Knick 提供完整的测量回路。自动化过程有助于提高标准化程度、避免记录错误并改善一致性。
使用 Knick cCare 系统能实现 FDA PAT 倡议的重要目标:
- 校准和清洁过程标准化,以减少波动和偏差
- 消除记录错误,从而减少错误批次
- 借助 AuditTrail 遵守现行法规
相关应用
您有疑问吗?我们竭诚为您服务。
联系我们